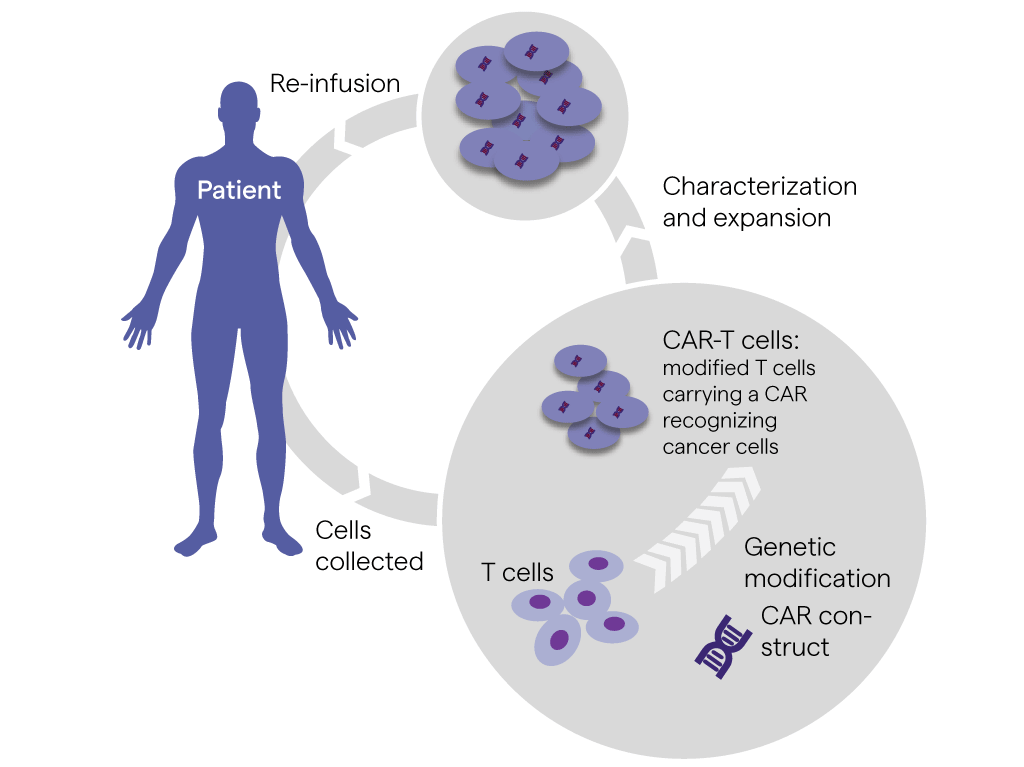
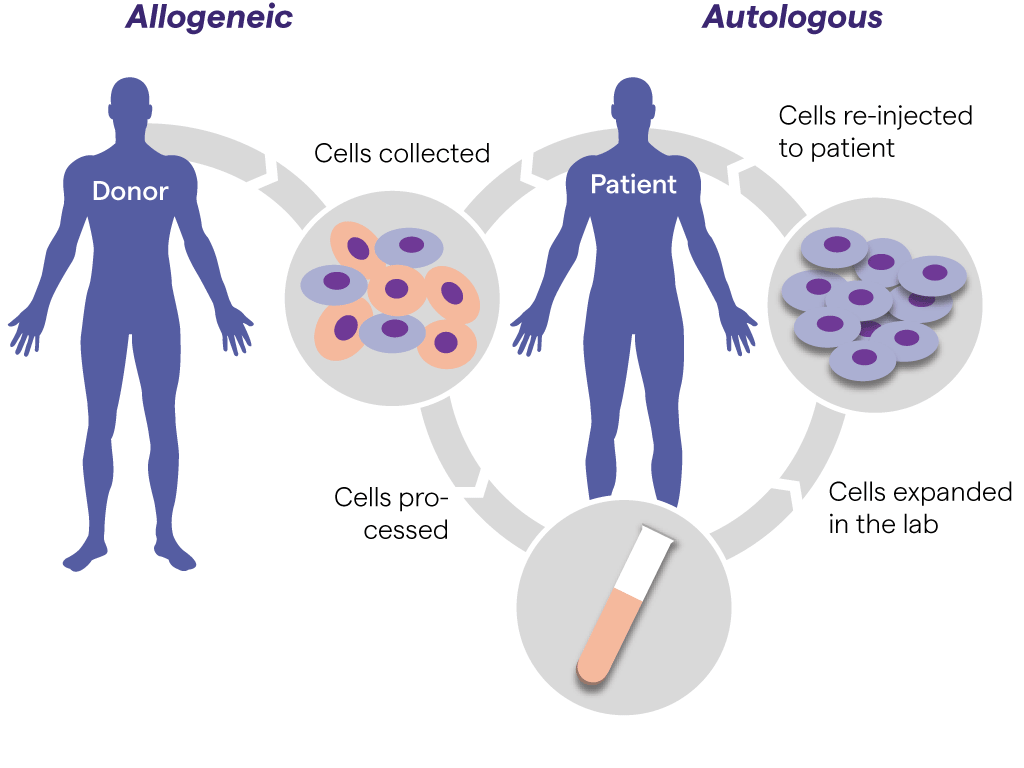
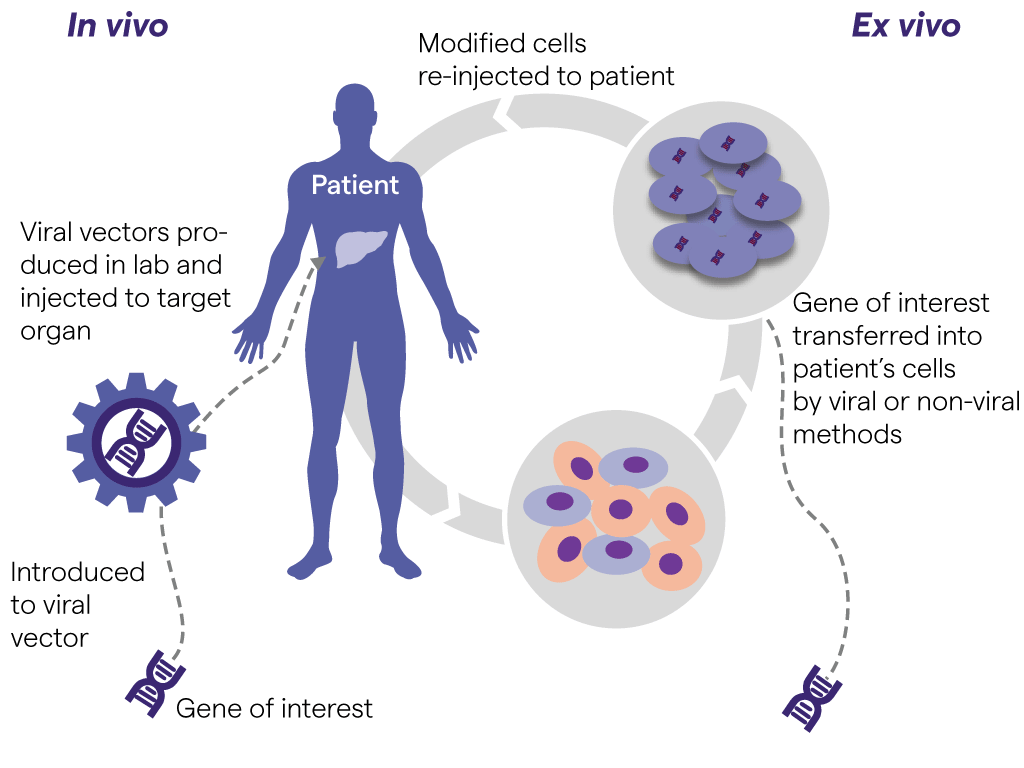
Gene therapy, using either in vivo or ex vivo strategies, holds promise for the delivery of enduring treatments or even cures for previously untreatable diseases (figure on the left side).
In vivo gene therapy involves the direct delivery of a modified and therapeutic gene into a patient – an approach that has achieved success in the treatment of neurological disorders and hemophilia.1 Some of the most encouraging applications of ocular gene therapy are for Leber congenital amaurosis (an inherited form of blindness), glaucoma, and age-related macular degeneration.
Ex vivo gene therapy (cell-based therapy) involves removing target cells from the body, modifying their genome in vitro and subsequently delivering these cells back to the patient. Ex vivo gene therapy applications have focused on immune cells such as T cells or NK cells to treat vari-ous diseases, particularly cancer.2
Owing to its targeted and personalized nature, ex vivo gene therapy benefits from an improved safety profile. However, cell retrieval and in vitro manipulation can be a costly and intricate process. In vivo gene therapy is potentially more straightforward, but faces challenges such as toxici-ty or the induction of immune responses.
Early clinical trial success with several treatments has resulted in approvals of five gene therapies to date, with numerous other clinical trials underway:
- Luxturna® in vivo gene therapy for RPE65 mutation-associated retinal dystrophy
- Imlygic® ex vivo gene therapy for melanoma
- Strimvelis® ex vivo gene therapy for severe combined immunodeficiency due to adeno-sine deaminase deficiency
- Yescarta® ex vivo gene therapy for diffuse large B-cell lymphoma and primary mediasti-nal large B-cell lymphoma
- Kymriah® ex vivo gene therapy for B-cell acute lymphoblastic leukemia and diffuse large B-cell lymphoma