For over 30 years, you have relied on Lonza for our bacterial endotoxin test (Bet) expertise. But we know that science is never finished. Our commitment to sustainability drives us forward, motivated by figuring out something that no one has before.
Worldwide, there is a growing appeal in the pharmaceutical industry for recombinant technologies that help alleviate, and eventually replace, the use of animal resources for endotoxin testing purposes. Adoption of recombinant test methods, like the PyroGene® rFC Assay, are on the rise. Increased use of recombinant methods has proven their equivalency to LAL-based methods and, therefore, increased confidence in implementing rFC methods in QC testing labs.
With the recombinant Factor C Assay, you can meet the demands of endotoxin testing while protecting a natural resource. Together, we safely bring your next medicine to life.
For a detailed product overview, educational materials, and scientific data about the effectiveness of rFC as a BET assay, please visit the PyroGene® rFC Product Page.
At Lonza, we are committed to protecting the welfare of the horseshoe crab through adopting best practices and actively supporting conservation efforts. To learn more about Lonza’s horseshoe crab conservation efforts please visit the Horseshoe Crab Conservation Page.
The PyroGene® rFC Assay contains the rFC enzyme cloned from the horseshoe crab. When endotoxin binds to recombinant Factor C, the activated rFC enzyme cleaves a synthetic fluorogenic substrate causing the solution to fluoresce. The recombinant Factor C assay works through a single enzymatic step and offers the same reliability as a LAL method – without the use of horseshoe crabs.
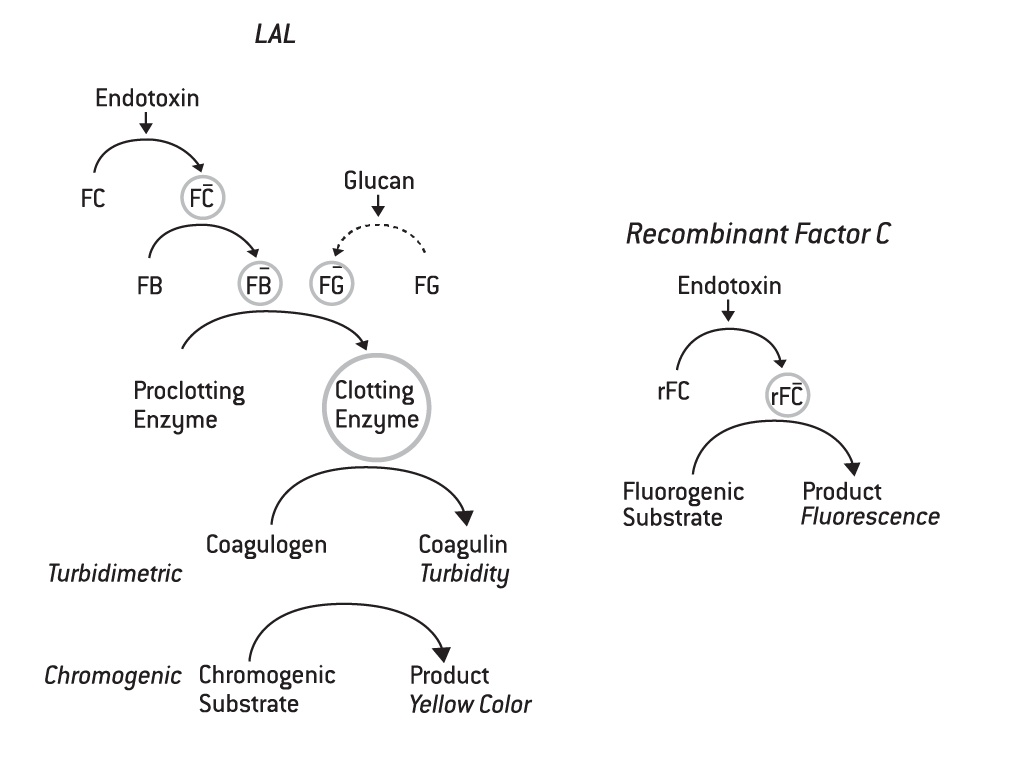
The image on the left illustrates the enzymatic cascade of the recombinant Factor C assay compared to the enzymatic activation pathway of traditional LAL assays, like the kinetic chromogenic and kinetic turbidimetric assays.
The PyroGene® rFC method is a quantitative assay that is run on a 96-well plate incubated in a microplate reader at 37°C that measures fluorescence with excitation/emission wavelengths at 380/440 nm. Due to the high dynamic range of the fluorescent signal, PyroGene® rFC delivers a quantitative range of 5.0 EU/ml – 0.005 EU/ml in a single step, with better resolution than conventional kinetic LAL assays.
Accepted by several global regulatory authorities including the FDA and the European Pharmacopeia, the recombinant Factor C method is a recognized, comparable endotoxin detection method to LAL-based assays. USP 28-NF 33 General Notices states that alternative testing methods, such as recombinant Factor C (rFC) can be used if the alternative method provides advantages in accuracy, sensitivity, precision, selectivity, or adaptation to automation.
According to USP, the rFC assay is still considered an “Alternative Test”, subject to the validation requirements of USP <1225> or ICH Q2B. Regulatory authorities will accept the test results of the recombinant Factor C assay, but a validation study must be performed for each product that will be tested using this method. (Validation studies are used to compare the alternative and compendial method, and verify the equivalence between the two methods of the assay. Post-validation, it is necessary to follow up with the appropriate regulatory filing for the drug product or device.)
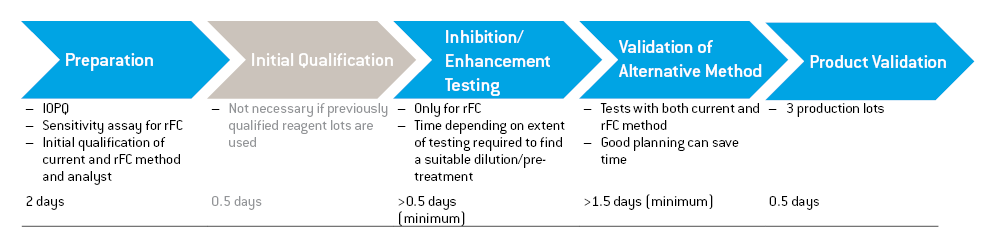
A possible validation scheme is outlined in the image on the left. The validation scheme is identical to that which would be needed for any LAL-based method with just the addition of one extra step, ”Validation of Alternative Method”.
We offer a full Validation Protocol that can be followed for your convenience. For further information, please submit your Validation Protocol Request below or contact our Scientific Support Team for a free consultation with our Subject Matter Expert.
PyroGene™ Validation Timeline
A possible validation scheme for the PyroGene™ rFC Assay can be accomplished in as little as five days, assuming that the product has been previously validated with a quantitative LAL method.
Endotoxin testing traditionally involves a number of manual laboratory preparation steps that are inherently prone to human error. To address these issues, we have created the PyroTec® PRO Automated Robotic Solution. Now compatible with the PYROGENT® 5000 Assay, Kinetic-QCL Assay and the PyroGene® rFC Assay, the PyroTec® PRO Solution provides laboratories the ability to reduce errors and increase throughput using rFC and LAL assays to suit all your testing needs.


PyroGene® Recombinant Factor C Assay Brochure
This brochure contains information on the benefits, history, and global footprint of the PyroGene® rFC assay.
PyroGene® Recombinant Factor C Assay Kit
View product:
PyroGene Test Kit – 192 tests